How is it possible for such a heavy and unstable element like nobelium to form stable compounds? What kinds of molecules does nobelium create when it reacts with water and nitrogen? Why does identifying these compounds matter for understanding the chemistry of the heaviest elements on the periodic table? Could studying nobelium’s behavior help clarify where it and other heavy elements fit in the periodic table? And what might we learn about the electronic structure and oxidation states of elements at the edge of the actinide series?
Could Nobelium Be the Heaviest Element Ever Seen Forming Real Compounds?
Related Encyclopedia

- 10028-14-5
- No
- 259.00000
- All (0)
- China (0)
- (0)
- 99644-37-8
- Br3No
- 498.71200
- All (0)
- China (0)
- (0)
- 72172-66-8
- H2No
- 261.01600
- All (0)
- China (0)
- (0)
- 99644-16-3
- NoO
- 274.99900
- All (0)
- China (0)
- (0)
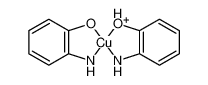
- 18347-30-3
- C12H11CuN2O2+
- 278.77400
- All (0)
- China (0)
- (0)
- 68002-60-8
- 0
- All (0)
- China (0)
- (0)
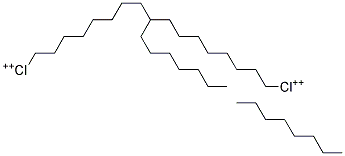
- 68424-95-3
- C32H66Cl2+4
- 521.77244
- All (0)
- China (0)
- (0)
- 13571-90-9
- C9H9Cl3O4
- 287.52400
- All (0)
- China (0)
- (0)
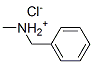
- 61789-73-9
- C8H12ClN
- 157.64058
- All (1)
- China (1)
- (1)
- 68956-79-6
- C25H46ClN
- 0
- All (1)
- China (1)
- (1)
Related Products More >
-
- 9002-86-2
- Request For Quotation
-
- 86508-42-1
- Request For Quotation
- Bulk
-
- 86508-42-1
- Request For Quotation
- 200KG/Drum
-
- 86508-42-1
- Request For Quotation
- bottle or customized
-
- 86508-42-1
- Request For Quotation
- Drum/Customized packaging
-
- 86508-42-1
- Request For Quotation
- 25KG/DRUM
-
- 86508-42-1
- Request For Quotation
- kg
-
- 86508-42-1
- Request For Quotation
- 25kg/Drum


 沪ICP备2021018848号-5
沪ICP备2021018848号-5


By studying nobelium’s chemistry, researchers hope to better understand the tricky question of which elements should be placed in group 3. Since nobelium fits the expected actinide pattern, it’s a solid piece of the puzzle. Next up, scientists want to look at heavier elements like lawrencium, rutherfordium, and dubnium to see how their chemistry evolves. Overall, these experiments help unlock the secrets of the heaviest elements, which have always been tough to study.
Identifying these compounds is crucial for understanding the chemistry of the heaviest elements on the periodic table. It provides direct evidence of their chemical behavior, which was previously difficult to confirm due to the short - lived nature of these isotopes. This helps clarify the position of these elements in the periodic table, especially regarding which end of the lanthanide and actinide series belongs in group 3.
Studying nobelium's behavior can indeed clarify its and other heavy elements' placement in the periodic table. It offers insights into preferred oxidation states, which are related to the element's position. Additionally, gas - phase chemistry in these experiments gives direct information about electronic orbitals and electron configuration accessibility.
Regarding the electronic structure and oxidation states of elements at the edge of the actinide series, this research can reveal trends and patterns. Unlike lighter elements with more well - defined properties, heavy elements like nobelium have complex electronic structures due to relativistic effects. A potential misunderstanding is that unstable elements cannot form stable compounds. However, in the right conditions, even unstable elements can react and form relatively stable molecular complexes, providing valuable chemical information.
When interacting with water, nobelium forms complexes incorporating hydroxide and water ligands; with nitrogen, it binds dinitrogen, creating cationic species observed in gas-phase experiments. Such interactions reveal its preferred oxidation states and electronic behavior, critical for understanding periodic trends at the actinide edge.
Identifying these compounds matters because they clarify how chemistry evolves in the periodic table’s outer reaches, resolving debates about group placement—particularly whether late actinides belong in group 3. Nobelium’s behavior, fitting expected actinide trends, guides predictions for heavier elements like lawrencium. Studying its electronic structure, via gas-phase chemistry, uncovers orbital configurations and accessible electron states, shedding light on oxidation state stability. This work not only solidifies nobelium’s position but also sets a precedent for characterizing heavier, more unstable elements, refining our grasp of periodicity itself.