I'm trying to understand the properties of water molecules. 1. Is water negatively charged? 2. What is the charge distribution in a water molecule? 3. How does this affect its interactions with other molecules? 4. Can water molecules form ionic bonds? 5. What are some examples of water's unique properties due to its charge distribution? Appreciate any help!!!
Is water negatively charged?
Related Encyclopedia
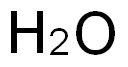
- 7732-18-5
- H2O
- 18.01
- All (50)
- China (13)
- (50)
- 53774-37-1
- OT2
- 24.03130
- All (50)
- China (13)
- (50)
- 32113-92-1
- HOT
- 22.02320
- All (50)
- China (13)
- (50)
- 32119-89-4
- HOT
- 21.02310
- All (50)
- China (13)
- (50)
- 32113-93-2
- DOT
- 23.02930
- All (50)
- China (13)
- (50)
- 32119-90-7
- DOT
- 22.02930
- All (50)
- China (13)
- (50)
- 20272-95-1
- DOT
- 21.02960
- All (50)
- China (13)
- (50)
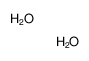
- 358616-29-2
- H3O2
- 35.02260
- All (50)
- China (13)
- (50)
- 61468-85-7
- OT2
- 23.03120
- All (50)
- China (13)
- (50)
- 12325-19-8
- H2O-
- 19.02320
- All (0)
- China (0)
- (0)
Related Products More >
-
- 9003-05-8
- USD 30.0000
- 25kg
-
- 9003-05-8
- USD 30.0000
- 25kg
-
- 1336-21-6
- CNY 500.0000
- 1ton


 沪ICP备2021018848号-5
沪ICP备2021018848号-5


Dipole Moment
The oxygen atom attracts electrons more strongly, creating a partial negative charge, while hydrogens have partial positive charges.
Neutrality Explained
These partial charges cause polarity but do not confer an overall negative charge to the water molecule.
Polar Nature
Water is a polar molecule because of the unequal sharing of electrons and bent shape, resulting in a partial positive charge near hydrogen atoms and a partial negative charge near oxygen.
Misconception
Although water has regions of partial charges, the molecule as a whole is not negatively charged; it balances out to neutrality.