How does the difference in electronegativity between carbon and oxygen specifically lead to the polarity of the carbon - oxygen bond? Do the number of valence electrons in carbon and oxygen play a role in determining bond polarity? In molecules containing these bonds, how does the polarity of carbon - oxygen bonds affect the overall molecular shape and properties? Also, are there any exceptions in chemical reactions where the expected polarity of these bonds might change? And how do solvents interact with molecules having non - polar carbon - carbon and polar carbon - oxygen bonds?
In Chemistry, Why Does Carbon Have a Non - Polar Bond with Itself but a Polar Bond with Oxygen in Terms of Polarity?
Related Encyclopedia
- 118090-85-0
- C3+
- 36.03210
- All (94)
- China (14)
- (94)
- 141219-98-9
- C36
- 432.38500
- All (94)
- China (14)
- (94)
- 129066-02-0
- C9
- 108.09600
- All (94)
- China (14)
- (94)
- 139055-01-9
- C11
- 132.11800
- All (94)
- China (14)
- (94)
- 145270-74-2
- C17
- 204.18200
- All (94)
- China (14)
- (94)
- 145270-72-0
- C15
- 180.16000
- All (94)
- China (14)
- (94)
- 145270-70-8
- C13
- 156.13900
- All (94)
- China (14)
- (94)
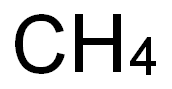
- 7440-44-0
- C
- 12.011
- All (94)
- China (14)
- (94)
- 12595-79-8
- C2+
- 24.02140
- All (94)
- China (14)
- (94)

- 121565-74-0
- C5
- 60.05350
- All (0)
- China (0)
- (0)
Related Products More >
-
- CNY Request For Quotation
-
- CNY Request For Quotation
-
- CNY Request For Quotation
-
- Request For Quotation
-
- 616-38-6
- CNY 3500.0000
- 1ton
-
- 124-38-9
- CNY 2500.0000
- 1ton
-
- 105-58-8
- CNY 2000.0000
- 1ton


 沪ICP备2021018848号-5
沪ICP备2021018848号-5


Now, about the number of valence electrons in carbon and oxygen and their role in bond polarity. Carbon has 4 valence electrons, and oxygen has 6. But the number of valence electrons doesn't directly determine bond polarity. The main thing is electronegativity. However, the valence electrons do play a role in how the atoms bond in the first place. Carbon can form different types of bonds to complete its outer shell, like single, double, or triple bonds. But when it comes to polarity, it's the difference in how strongly the atoms pull on the shared electrons, not just the number of valence electrons.
In molecules with carbon - oxygen bonds, the polarity of these bonds really affects the overall molecular shape and properties. Take water for example, which has oxygen - hydrogen bonds, a similar polar situation. The polar bonds in water make the molecule have a bent shape. In organic molecules with carbon - oxygen bonds, like ethanol (C₂H₅OH), the polar carbon - oxygen bond makes the molecule have a certain dipole moment. This affects things like solubility. Polar molecules like ethanol can dissolve in polar solvents like water because the positive end of one molecule (the carbon - hydrogen part in ethanol) is attracted to the negative end of water, and vice versa. Also, the polarity can affect boiling points. Molecules with polar carbon - oxygen bonds often have higher boiling points because the molecules are attracted to each other more strongly due to these polar forces.
As for exceptions in chemical reactions where the bond polarity might change, well, there are some cases. In some complex organic reactions, when there are strong electron - withdrawing or electron - donating groups nearby, the electron distribution in the carbon - oxygen bond can change. For example, in a reaction where a carbonyl group (C=O) is next to a highly electronegative atom or a group that can pull electrons away, the carbon - oxygen bond might become even more polar or less polar depending on the situation. But these are more advanced chemical scenarios.
Finally, solvents interact differently with molecules having non - polar carbon - carbon and polar carbon - oxygen bonds. Non - polar solvents, like hexane, are made up of non - polar molecules. They interact well with non - polar carbon - carbon - rich molecules through weak van der Waals forces. It's like two people who are not very clingy just hanging out together. But polar solvents, like water, are attracted to polar carbon - oxygen - containing molecules. The positive and negative ends of the polar solvent molecules interact with the partial charges on the polar carbon - oxygen bonds. So, if you have a molecule with both non - polar carbon - carbon parts and polar carbon - oxygen parts, like a fatty acid, it can have a complex interaction with solvents. The non - polar part will be more attracted to non - polar solvents, and the polar part to polar solvents. This is why fatty acids can form structures like micelles in water, where the non - polar tails are grouped together away from the water, and the polar heads are facing the water.
The number of valence electrons in carbon and oxygen also plays a role. Carbon has four valence electrons and oxygen has six. In a carbon - oxygen bond, the oxygen's higher electronegativity, combined with its relatively larger number of valence electrons, contributes to its ability to attract the shared electrons more strongly, further enhancing the bond's polarity.
In molecules with carbon - oxygen bonds, the polarity of these bonds affects the overall molecular shape and properties. For example, in a carbonyl group (C=O), the polarity of the bond influences the molecule's reactivity and its interactions with other molecules. It can also affect the molecule's dipole moment, which in turn impacts its physical properties like boiling point and solubility.
There are exceptions in chemical reactions where the expected polarity of carbon - oxygen bonds might change. For instance, in some complex organic reactions involving multiple functional groups, the electron density distribution can be altered, potentially changing the polarity of the carbon - oxygen bond.
When it comes to solvents, polar solvents tend to interact more strongly with molecules having polar carbon - oxygen bonds due to dipole - dipole interactions. Non - polar solvents, on the other hand, interact better with non - polar carbon - carbon bonds. In molecules with both non - polar carbon - carbon and polar carbon - oxygen bonds, the solvent may interact differently with different parts of the molecule, affecting the molecule's behavior in the solution.
The number of valence electrons in carbon and oxygen does play a role. Carbon has four valence electrons, and oxygen has six. When they bond, they share electrons, but because oxygen is so electronegative, it pulls those shared electrons closer to itself, making the bond polar.
In molecules with carbon-oxygen bonds, this polarity affects the overall shape and properties. For example, in water (H2O), the polar carbon-oxygen bonds make the molecule bent, and give it its unique properties like being a good solvent and having a high boiling point.
There aren't really exceptions where the expected polarity of these bonds changes in normal chemical reactions. But in some really weird, extreme conditions, like in a particle accelerator or something, who knows what could happen!
Solvents interact differently with molecules having non-polar carbon-carbon bonds and polar carbon-oxygen bonds. Non-polar solvents, like oil, don't really mix with polar molecules because they can't break those strong polar bonds. But polar solvents, like water, can dissolve polar molecules because they can form hydrogen bonds or other interactions with the polar carbon-oxygen bonds. So, the polarity of the bonds really determines how a molecule will interact with its environment.